

Double the results with less effort
Our studies show that children treated with Evira achieve significantly greater weight loss after one year compared to traditional methods - doubling the results while using fewer resources than treatments recommended by the USPSTF.
The chart below illustrates the reduction in BMI SDS score, a key measure of childhood obesity, among randomnly selected patients during their first year with Evira.
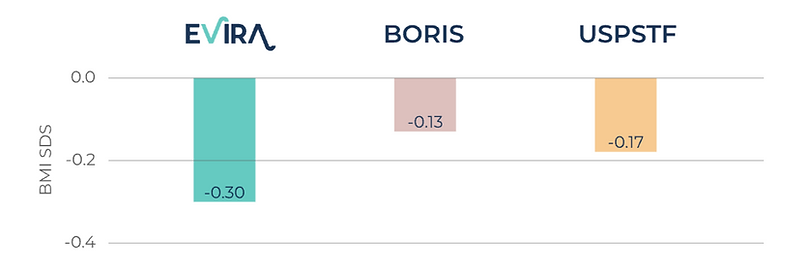
Comparison between treatment results in WHO BMI SDS after one year of treatment with Evira and traditional treatment according to the Child Obesity Register in Sweden (BORIS) as well as results presented in a meta-study for children who received at least 26 hours of professional support over a year compiled by
Proven superior in clinical studies
Validated in rigorous trials, Evira consistently delivers better outcomes than traditional pediatric obesity treatments.

Happier families
After one year with Evira, 78% of families were satisfied with the treatment results—significantly higher than the 11% satisfaction rate in traditional treatment.

Fewer canceled visits
Evira significantly reduced appointment cancellations by 45% over a six-month period. Only 40% of Evira patients canceled at least one visit, compared to 85% with traditional treatments.

Lower cost
Evira delivers unparalleled outcomes in pediatric obesity treatment—without the need for medications or surgery. Children treated with Evira experience twice the effectiveness on average compared to those receiving traditional treatments.
Research about Evira
Evira has been evaluated clinically and in a research context in various forms for several years. Treatment with Evira gives, on average, twice as good an effect compared to traditional treatment.
One-year study in clinical practice
In our latest one-year study together with prominent researchers from Karolinska Institutet, the first 107 patients at the Martina Children's Hospital Center for Weight Health were able to use Evira's platform and treatment method for a year. A matched control group of 321 patients was randomly selected from the national quality registry BORIS who received traditional childhood obesity treatment during the same time period. The intervention group using Evira had a relative weight loss of 0.30 WHO BMI SDS units, compared to the control group which averaged 0.15 WHO BMI SDS units.

Mean change in WHO BMI SDS for Evira patients (dark gray) and the control group of BORIS patients (light gray) after 1 year of treatment divided by sex, age and degree of obesity in the 1-year study.
In addition to being able to see twice as good treatment results with Evira compared to traditional treatment, we were able to see a big effect on teenagers - a treatment group that has been very difficult to reach before with traditional treatment.
Feasibility study
In Evira's early days, the platform was tested in a small feasibility study at three clinics over a period of six months. The study showed a good treatment effect after six months compared to traditional treatment and showed that patients and therapists were very satisfied with the platform.
.png)
Change in WHO BMI SDS from baseline to follow-up at three and six months for intervention and control groups in the feasibility study.
Almost everyone in the intervention group who used Evira reported that Evira helped them reach their treatment goals, and that it was easier to get in touch with therapists at the clinic. The families who used Evira were significantly more satisfied with the treatment results compared to the control group.
Doctoral thesis by Linnea Johansson
Linnea Johansson, Ph. D, has researched mobile health interventions with Evira as the main focus in his doctoral thesis . She brings up several important lessons about how to think about digital treatment and analyzes what worked and what didn't work during Evira's development period. She concludes by stating that digital health interventions together with clinical visits, such as Evira, provide a better treatment effect than behavioral treatment alone. Linnea also places great emphasis on the importance of technical maturity of digital systems, as well as the importance of teaching clinical staff how treatment with the help of digital support systems should be carried out.
EurEvira - international multicenter study
(ongoing)
EurEvira is an international multicenter study that is ongoing in several European countries to evaluate how Evira together with local childhood obesity treatment works in countries other than Sweden.
The study is currently being conducted at the following sites in Europe.
Published research
”Effect of an interactive mobile health support system and daily weight measurements for pediatric obesity treatment, a one-year pragmatical clinical trial”, Emilia Hagman, Linnea Johansson, Claude Kollin, Erik Marcus, Andreas Drangel, Love Marcus, Claude Marcus, Pernilla Danielsson, International Journal of Obesity, online 31 maj 2022, doi: 10.1038/s41366-022-01146-8.
Thesis for doctoral degree(Ph.D) 2022. Mobile Health interventiopns and cardiorespiratory fitness in pediatric obesity.Linnea Johansson, Karolinska Institutet.
A novel interactive mobile health support system for pediatric obesity treatment: a randomized controlled feasibility trial. Linnea Johansson, Emilia Hagman, Pernilla Danielsson. BMC Pediatr. 2020;20:447.
